EAHP EU Monitor - 13 February 2015
 The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.You can subscribe to receive the EAHP EU Monitor by email here.
 The state of hospital pharmacy in Europe in 2015: EAHP opens new look practice survey!
The state of hospital pharmacy in Europe in 2015: EAHP opens new look practice survey!
In the run up to its annual Congress, the European Association of Hospital Pharmacists (EAHP) has issued a new format practice survey to heads of hospital pharmacy across Europe. The survey aims to shine light on the current state of practice in EAHP's member countries, and where attention for practice development is most required. More information here.
 EU agencies call for improved data on antimicrobial consumption in hospitals
EU agencies call for improved data on antimicrobial consumption in hospitals
 EU agencies call for improved data on antimicrobial consumption in hospitals
EU agencies call for improved data on antimicrobial consumption in hospitals Three agencies of the European Union have teamed together to publish a joint report that calls for improved data on antimicrobial consumption in hospitals in more European countries, amongst other recommendations.
The report from the European Medicines Agency (EMA), European Centre for Disease Prevention and Control (ECDC) and European Food Safety Authority (EFSA) was published at the end of January 2015. Titled 'The ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals', the study was carried out at the request of the European Commission.
The report was formed on the basis of 2011 and 2012 monitoring data from 5 EU monitoring networks under the remit of the 3 agencies. This includes ESAC-NET, a Europe-wide network of national surveillance systems coordinated by ECDC providing independent reference data on antimicrobial consumption in EU Member States, Iceland and Norway. It collects and analyses antimicrobial consumption data from the community (primary care) and from hospitals.
The joint report highlighted that while in most countries antibiotic consumption is collected separately in primary care and secondary care, some countries (1/3 of ESAC-NET members) are not able to split the data. This leads to difficulties for ESAC-NET in gaining a full understanding of antibiotic consumption trends in Europe. ESAC-NET is endeavouring to make changes to improve this scenario. Specific data on hospital antibiotics consumption is missing for 7 states in the report: Austria, Germany, Poland, UK, Check Republic, Spain and Hungary.
Other findings included:
- Comparison of antimicrobial consumption data in animals and humans in 2012, both expressed in milligrams per kilogram of estimated biomass, revealed that overall antimicrobial consumption was higher in animals than in humans, although contrasting situations were observed between countries.
- In both humans and animals, positive associations between consumption of antimicrobials and the corresponding resistance in bacteria were observed for most of the combinations investigated. In some cases, a positive association was also found between antimicrobial consumption in animals and resistance in bacteria from humans.
The report will be used to inform the European Commission's Action Plan on antimicrobial resistance, and will become a regular series of collaborative reports by the 3 EU agencies.
Press release here.
Full report here.
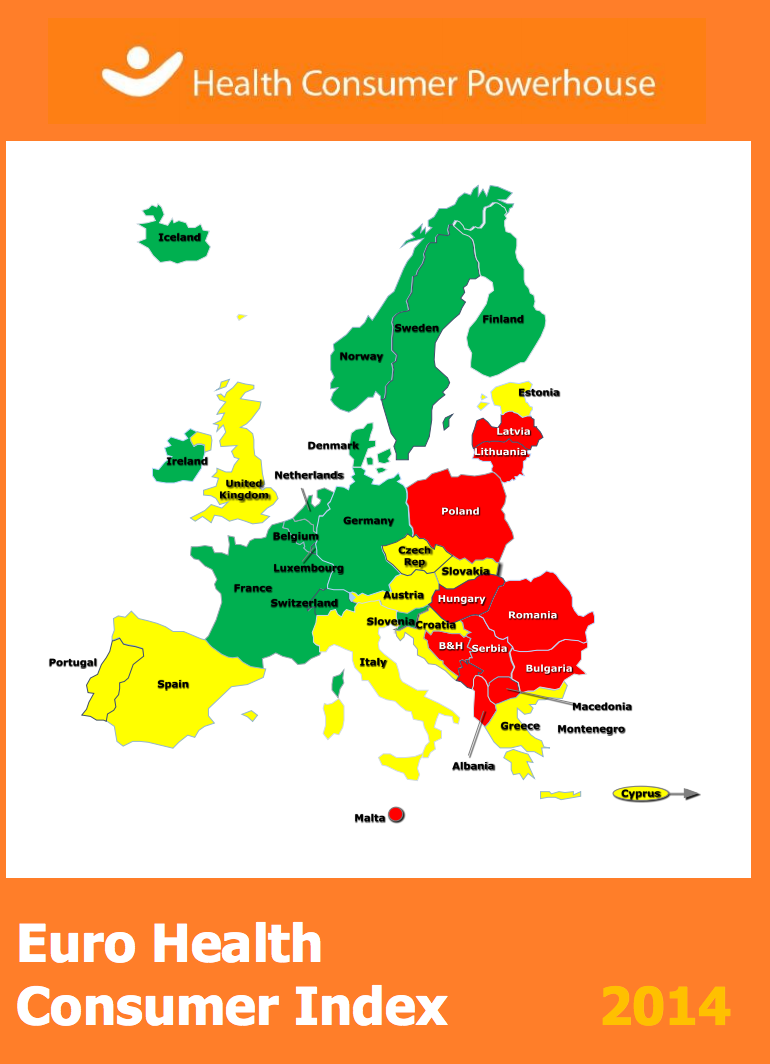 Euro Health Consumer Index continues to suggest Netherlands as the best health care system in the EU
Euro Health Consumer Index continues to suggest Netherlands as the best health care system in the EU
The Netherlands has retained its position at the top of the annual Euro Health Consumer Index (EHCI). The Index ranks healthcare systems in Europe on 48 indicators, including patients' rights and information, accessibility, prevention and outcomes. The Netherlands secured a top position among 36 European countries for the fifth year in a row, scoring 898 of a maximum 1,000 points.
Within the EU, the Netherlands was followed by Finland, Denmark, and Belgium as the countries with the best healthcare, while Romania, Lithuania and Poland scored the lowest. In an overall comparison in Europe, Switzerland came second with Norway in third place.
Responding to the publication of the Index, EU Health Commissioner Vytenis Andriukaitis commented that whilst there could be other ways to measure and rank healthcare systems, he welcomed the efforts towards ranking health systems as "it stimulates discussion on healthcare systems and performance assessment".
The Lithuanian Commissioner, whose country got the second-lowest place in the survey among EU member states, said that the Commission is interested in seeing more healthcare comparisons so that member states can learn best practices from each other.
In relation to hospital specific aspects:
- Denmark is congratulated for having a hospital registry on the Internet showing which hospitals have the best medical results;
- Germany is considered to be making changes to its hospital system to enable more specialized hospitals to emerge
- Scotland is criticized for not making hospital level outcome information available to the public, in contrast to NHS England
- NHS England, Bulgaria and Poland are praised for reducing resistance hospital infections
News report here.
Full report, and country specific breakdowns, here.
 EJHP: Open Access article on stability of octreotide acetate
EJHP: Open Access article on stability of octreotide acetate
An open access article recently published in the European Journal of Hospital Pharmacy (EJHP) examines the critical concentration of sodium bisulfate needed to preserve the stability of octreotide using actual drugs containing sodium bisulfate.
The article was submitted from the Toyama University Hospital in Japan and found that Octreotide concentrations decreased significantly at a sodium bisulfate concentration of 0.1 mg/mL or higher after 10 days when octreotide was mixed with sodium bisulfate solutions at various concentrations
Full article here.
______________________________________________________________________________________________
 Currently open consultations of hospital pharmacy interest
Currently open consultations of hospital pharmacy interest
European Medicines Agency consultation on application of transparency rules of EU Clinical Trial Regulation – deadline 18th February. More information here.
US Department of Health and Human Services (HHS) and National Institutes of Health (NIH) consultation on expanded reporting obligations for funded clinical trials – deadline 19th February. More information here.
European Commission consultation on the Working Time Directive – deadline 15th March. More information here.
______________________________________________________________________________________________
 Congress preview: Peggy Maguire keynote on patient empowerment
Congress preview: Peggy Maguire keynote on patient empowerment
Ahead of EAHP's 2015 Congress in Hamburg (25th-27th March), EAHP's EU Monitor looks forward to the first keynote speech from Peggy Maguire, President of the European Public Health Alliance and Director General of the European Institute of Women's Health. The topic of the keynote will be patient empowerment through education, and will explore how in modern health systems the role of patient goes much beyond being a subject of health care. The speech will investigate the education needs for patients in terms of medication efficacy and side effects, and will be informative to hospital pharmacists in respect of their counseling role with patients.
Ms Maguire has been a member of the EU Commission's External Advisory Group on Ageing and Disability, and WHO expert group on gender mainstreaming. Publications include: Women's Health in Europe-Facts and Figures across the EU; Reducing Health Inequalities and a series of policy briefs on chronic diseases and health issues affecting women. Peggy's previously worked as Director of Development at the National Maternity Hospital, Dublin and Director of the Research and Education Foundation at the Irish College of General Practitioners.
More information here.




