EAHP EU Monitor - latest news - 5 February 2014

You can subscribe to receive the EAHP EU Monitor by email here.
.jpg) What are we measuring in European Hospital Pharmacy?
What are we measuring in European Hospital Pharmacy?
As part of its Summit project to define the future of hospital pharmacy in Europe, EAHP has launched a survey of senior pharmacists within hospitals to better understand what aspects of service are currently measured.
More information here.
European Commission launches a joint action on chronic diseases and multi-morbidity
The European Commission has brought together 38 organisations from 22 EU Member States, Norway and Iceland with the purpose of collecting, validating, scaling up and transferring good practices to address chronic diseases, multi-morbidity and diabetes..
The third work package of the joint action focuses on ‘the development of common guidance and methodologies for care pathways for multi-morbid patients’. The focus of this work package will be on outcome measures - interventions to promote the provision of high quality care that meets the needs of people with multimorbidity.
The Commission believe that that past actions on this issue have largely focused on analysis of the impact of multimorbidity on individuals (and healthcare systems), with very few initiatives examining which interventions have helped improve outcomes.
It is expected that the work package will provide a comprehensive review of existing work on care pathway interventions addressing multimorbid patients across EU - based on clinical evidence. It will generate a thorough repository of good practices and clinical data on the effectiveness of care interventions tackling chronic multimorbidity conditions, including polypharmacy.
The action is being led by Juan E. Riese PhD MBA of the Institute of Health Carlos III in Madrid, Spain.
More information here.
Information on the multi-morbidity work package here.
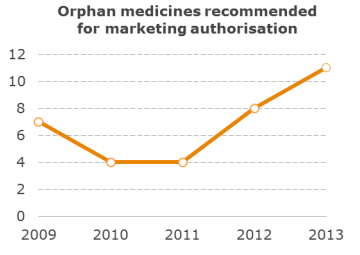 Orphan drug marketing authorisations continue to increase
Orphan drug marketing authorisations continue to increase
A total of 11 out of 81 medicines recommended for marketing authorisation by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) in 2013 were intended for the treatment of rare diseases, continuing a year on year increase (8 in 2012 and 4 in 2011).
IOne of the explanations offered by the Committee for Orphan Medicinal Products (COMP), the EMA committee responsible for recommending orphan designation of medicines for rare diseases, is that developers of orphan medicines are now making much better use of the tools offered by the Agency and the European Orphan Regulation to support them in the development of their medicines.
“12 years after the European Orphan Regulation came into force, it is undoubtedly serving its purpose, with more and more orphan medicines reaching patients with rare diseases each year," explains Bruno Sepodes, Chair of the COMP.
More information here.
In other news, as part of its drive to improve the transparency of its decision making processes, the EMA have begun publishing the full minutes of the Committee for Medicinal Products for Human Use (CHMP), the Committee for Medicinal Products for Veterinary Use (CVMP) and the Committee for Advanced Therapies (CAT).
More information here.
 Congress preview: hospital pharmacy into primary care
Congress preview: hospital pharmacy into primary care
Over the last few years health-care systems have begun to evolve to enhance the pharmaceutical management of patients as they move through the interfaces of care and in particular as they transit between secondary and primary care. Pharmacy related services in each sector must adapt to ensure appropriate support of patients during transfer of care.
In response to these changes, hospital pharmacists have developed services, through a process of innovation and translational research, which supports individualised pharmaceutical care and efficient drug therapy management within primary care.
The seminar at the 19th Congress of the EAHP on this subject will take place on Wednesday 26th March 2014 (2pm to 3.30pm) and Thursday 27th March (0830-1000am).
Information on the seminar here.
Information on the full Congress programme here.
Journal highlight: Editor Phil Wiffen debates medication reviews and the available evidence
In an editorial piece for the latest edition of the European Journal of Hospital Pharmacy (EJHP), Editor-in-Chief Phil Wiffen debates the issues provoked by a 2013 Cochrane review of the role played by medication reviews.
The Editor advises: “what can seem like evidence that something does not work can often be just a lack of evidence”, going on to highlight the need for pharmacists to demonstrate effectiveness and value in the roles they perform.
A future edition of the EHJP in 2014 will focus on medicines review.
Full article here.


.gif)



