EU Monitor –5th anniversary of the European Statements
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
You can subscribe to receive the EAHP EU Monitor by email HERE.
EAHP celebrates 5thanniversary of the European Statements

Throughout the month of May, the European Association of Hospital Pharmacists (EAHP) celebrated the 5th anniversary of the European Statements of Hospital Pharmacy. The European Statements express commonly agreed objectives which every European health system should aim for in the delivery of hospital pharmacy services. Their implementation is being carried out within EAHP’s member countries with the support of the Statement Implementation ambassadors.
To facilitate the move towards Statement Implementation, EAHP launched a self-assessment tool and the Statement Implementation Learning Collaborative Centres (SILCC) programme. The online self-assessment tool allows hospital pharmacists to assess the level of implementation of the European Statements within their hospitals, while the SILCC programme provides hospital pharmacists with the opportunity to visit hospitals from other EAHP member countries to learn about pharmacy procedures linked to the European Statements. With the help of both of these tools, EAHP has already significantly increased the awareness as shown in the surveys of hospital pharmacy practice.
To further improve patient outcomes throughout Europe, EAHP actively works on increasing the implementation of the European Statements in all its member countries. In particular the self-assessment tool is a great instrument to increase uptake at local level. The next free online training session on the self-assessment tool will be organised on Tuesday, 2nd July 2019 at 17h30 CEST. All hospital pharmacists wanting to learn more about the tool are welcome to join. Registration is possible via statements[at]eahp[dot]eu.
Learn more about the European Statements HERE
Access the self-assessment tool HERE
Learn more about the SILCC programme HERE
Medical devices - Factsheet for Healthcare Professionals and Health Institutions
Earlier this month, the European Commission published a factsheet for healthcare professionals and health institutions dealing with the new Medical Device Regulations. The document – which has also been translated into the official languages of the EU – touches on the difference between the old regime and the new Medical Devices Regulation(2017/745/EU) as well as the new In Vitro Diagnostic Medical Devices Regulation(2017/746/EU). The implementation of both Regulations will progress gradually starting in May 2020 for medical devices and in May 2022 for in-vitro diagnostic devices.
The new Regulations aim at strengthening the regulatory regime for devices by
- Widening and clarifying the scope of the legislation, including for example, aesthetic implants and medical software;
- Ensuring a closer supervision of the notified bodies by the national authority;
- Offering more responsibility and authority to the assessment bodies, including regular checks on manufacturers and thorough testing;
- Clarifying the rights and obligations of the parties involved in the supply chain, covering also internet sales and diagnostic services;
- Ensuring transparency to patients and healthcare professionals through a database (EUDAMED) containing comprehensive information about the devices available on the EU Market;
- Introducing a Unique Device Identification system (UDI system) to ensure post-marketing surveillance and traceability;
- Implementing stricter requirements for clinical evidence;
- Adapting the safety and performance requirements to new health technologies; and,
- Enhancing coordination between national surveillance authorities and aligning to international guidelines to facilitate trade.
The factsheet covers aspects of these changes that are relevant for healthcare professionals, such as for example clinical investigations, obligations and regulatory requirements of economic operators, CE marking, traceability, the EUDAMED database and reprocessing of single-use medical devices. In addition, the document contains some frequently asked questions.
Access the factsheet HERE
Finnish council presidency plans to tackle occupational health and safety

In a couple of days, Finland will take over the rotating Council presidency from Romania. Ahead of this change, Finland published its programme for the next six months which touches on the economic well-being in Europe and fostering growth. Finland aims at achieving its goals through an increase of skilled workers in the EU, in particular by establishing more routes for legal migration, improving labour mobility and increasing women’s participation in the workforce.
The programme focuses on four key priorities which include
- strengthening of common values and the rule of law;
- making the EU more competitive and socially inclusive
- strengthening the EU’s position as a global leader in climate action; and
- protecting the security of citizens comprehensively.
Health is covered in the second point, in particular the promotion of occupational health and safety within the competences of the EU and the digitalisation of the sector.
Read the full presidency programme HERE
Electronic cross-border health services move to the next phase
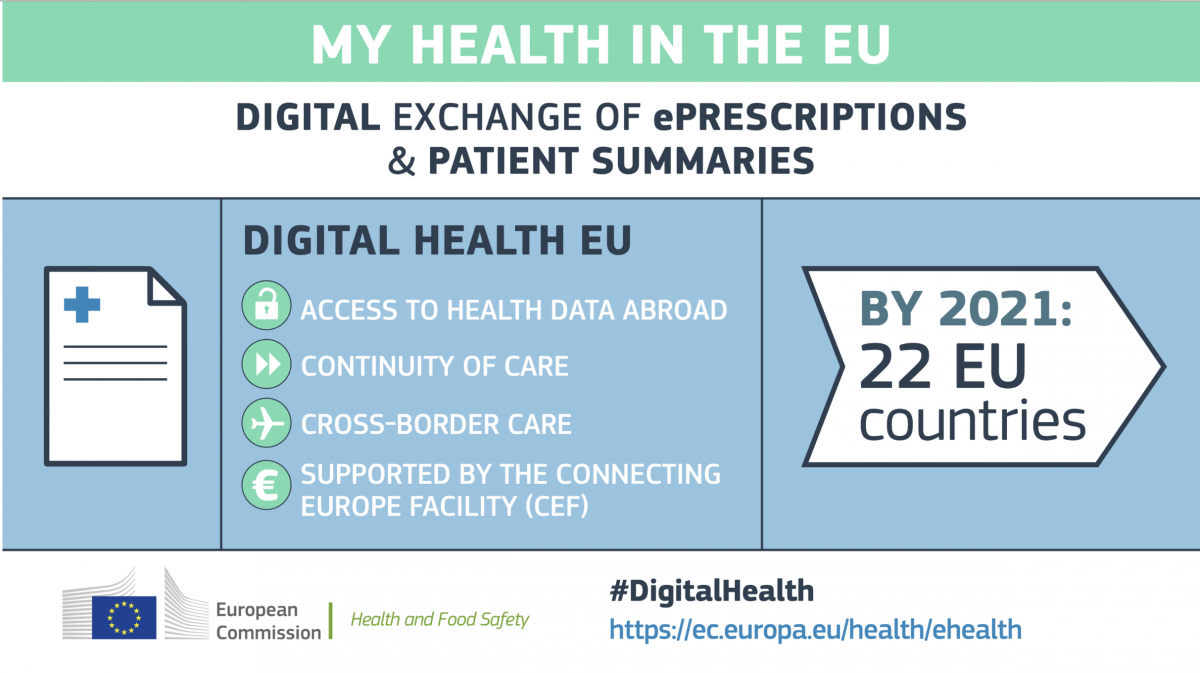
In the EU Monitor of 17thApril 2019, EAHP reported on the growth of the ePrescription network. The electronic cross-border health service initiative of the European Commission aims at facilitating the exchange of patient summaries and ePrescriptions. By 2021, these services should be gradually implemented by 22 EU countries, namely Austria, Belgium, Croatia, Cyprus, Czechia, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Slovenia, Spain and Sweden.
The implementation of these services is supported by the new eHealth Digital Service Infrastructure (eHDSI). The eHDSI is the initial deployment and operation of services for cross-border health data exchange. It is financed by the Member States and the European Union. The infrastructure has been rolled-out in 4 countries. These are currently offering the following exchanges:
- Finland: ePrescriptions of outgoing travellers
- Estonia: ePrescriptions of incoming travellers
- Luxembourg: Patient Summaries of incoming travellers
- Czech Republic: Patient Summaries of outgoing travellers
- Croatia: ePrescriptions of incoming travellers
Learn more about electronic cross-border health services HERE
EJHP: Systematic review of stability data pertaining to selected antibiotics used for extended infusions in outpatient parenteral antimicrobial therapy

The online first edition of the European Journal of Hospital Pharmacy (EJHP) recently published a systematic review of stability data pertaining to selected antibiotics used for extended infusions in outpatient parenteral antimicrobial therapy (OPAT) at standard room temperature and in warmer climates. The analysis underlines the lack of sufficient stability data of antibiotic use in warmer climates and consequently calls for studies that verify the stability and appropriate use of many antibiotics used in OPAT at standard room temperature.
Read the review HERE
Interested in becoming a SILCC host?
In March 2018, EAHP launched its Statement Implementation Learning Collaborative Centres (SILCC) programme. The SILCC programme allows hospital pharmacists to visit hospitals (SILCC hosts) from other EAHP member countries to learn more about pharmacy procedures linked to the European Statements of Hospital Pharmacy. Hospitals from Germany, Hungary, Italy, Spain, Sweden, Turkey and the United Kingdom are already participating. SILCC hosts are hospitals from EAHP member countries willing to provide training in. Prior to becoming a SILCC host, hospitals will need to assess their pharmacy using EAHP’s self-assessment tool. In case the description fits to your hospital, apply and become a SILCC host. More information is available on the Statement website or via statements[at]eahp[dot]eu.
------------------------------------------------------------------------------------------------

Consultations
Public consultation on EMA Regulatory Science to 2025
The purpose of this public consultation is to seek views from EMA’s stakeholders, partners and the general public on EMA’s proposed strategy on Regulatory Science to 2025 and whether it meets stakeholders’ needs. By highlighting where stakeholders see the need as greatest, participants have the opportunity to jointly shape a vision for regulatory science that will in turn feed into the wider EU network strategy in the period 2020-25.
Deadline – 30th June 2019
Access consultation HERE
EMA - Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections
The EMA has launched a consultation on the revision of its guideline on the evaluation of human medicines indicated for the treatment of bacterial infections. Antimicrobial resistance is a global public health problem. Regulators in the European Union, the United States and Japan have had extensive discussions over the last few years to explore and agree how to align as much as possible their respective data requirements so that medicine developers can design clinical trials that meet the evidence needs of multiple regulatory agencies. The revised guidance reflects the outcome of these discussions.
Deadline – 31st July 2019
Access consultation HERE
EMA – Public consultation on key principles for the electronic product information of EU medicines
The European Medicines Agency (EMA), together with the European Commission (EC), has launched a public consultation on draft key principles which will form the basis on which the electronic product information (ePI) for human medicines will be developed and used throughout the European Union. The rationale behind the ePI is that digital platforms open additional possibilities to disseminate the PI electronically. This can address some of the current limitations and better meet patients’ and healthcare professionals’ needs for accessible, up-to-date information on medicines. The draft key principles are the result of extensive discussions and consultations carried out by EMA, the Heads of Medicines Agencies (HMA) and the EC throughout 2018, with representatives of all stakeholder groups.
Deadline – 31st July 2019
Access consultation HERE





