EU Monitor -Apply for membership in EAHP’s Scientific Committee!
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
You can subscribe to receive the EAHP EU Monitor by email HERE.
EAHP’s Scientific Committee is looking for a new member

The European Association of Hospital Pharmacists (EAHP) has launched a call for the expression of interest addressed to all hospital pharmacists and scientists who wish to be considered for membership in EAHP’s Scientific Committee. This call for expressions of interest closes on 15 December 2019.
EAHP is committed to providing educational innovation and training of hospital pharmacists to a level of specialisation and maintain continuing professional development. The responsibility of facilitating and enhancing the professional growth of European hospital pharmacists and developing hospital pharmacy in order to promote the best and safest use of medicines and medical devices for the benefit of patients in Europe lies with EAHP’s Scientific Committee.
Members of the Scientific Committee strive to
- identify the educational needs of EAHP members and prepare educational programmes to meet those needs;
- provide knowledge and application based educational programmes to assist pharmacists who practice in hospitals meet their patient care responsibilities and expand their professional roles and goals;
- share best practice, innovation and educational programmes that can be applied to daily practice; and,
- promote hospital pharmacy practice research.
More information on the responsibilities of the Scientific Committee, the selection criteria and the application process are available on EAHP’s website.
Access the call for the expression of interest HERE
State of Health in the EU suggests task shifting
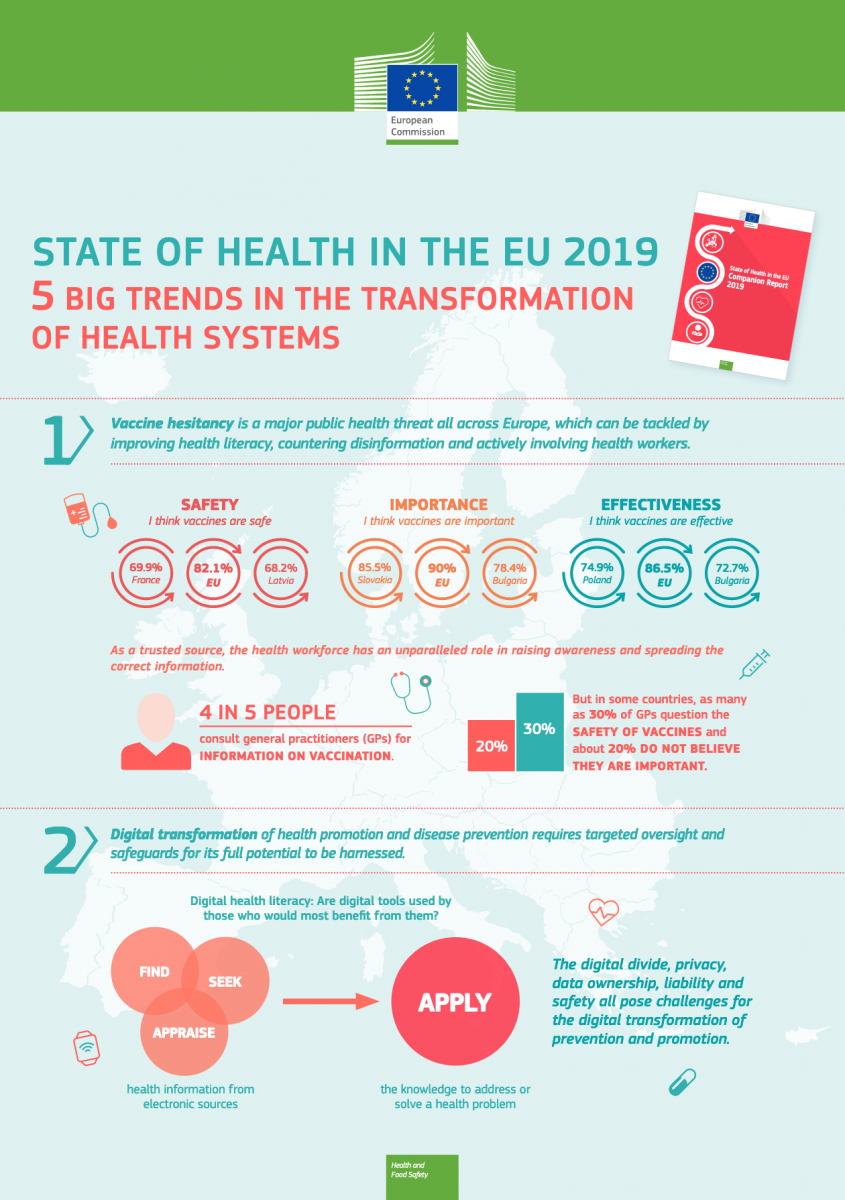
In late November, the European Commission published its newest addition to the State of Health in the EU cycle. The 2019 companion report summarises information of 30 different European countries. This report is accompanied by in-depth country profiles which depict an analysis of the health system taking into account population health, risk factors, effectiveness, accessibility and resilience.
In terms of trends, the companion report highlights vaccine hesitancy, digital health, task shifting as well as affordable, innovative and sustainable medicines as key themes. Health literacy, countering disinformation and actively involving health workers are listed as measures that should be used to combat vaccine hesitancy which was identified as a major public health threat all across Europe. In relation to the digital transformation it was stressed that more needs to be done to harness the full potential, while at the same time taking into account oversight and data security.
Access to affordable care was again identified as a gap both in terms of clinical needs and socioeconomic characteristics of patients. To this end the companion report underlines that policymakers struggle to balance accessibility, incentives for pharmaceutical innovation and the fiscal sustainability of health spending and that evidence to measure the quality of affordable, preventive and curative health care is missing. Concerning the resilience of health systems the report suggests to expand the roles of nurses and pharmacists through task shifting.
Read the companion report HERE
Access the individual country profiles HERE
Expand your knowledge in the field of emergency and disaster pharmacy
In 2018 a study was carried out on the initiative of Prof Pascal Bonnabry (Chief-pharmacist at the pharmacy of Geneva University Hospitals) and Dr Nicolas Widmer (Chief-pharmacist at the pharmacy of Eastern Vaud Hospitals), who were mandated by the Swiss Confederation to develop a Centre for Emergency and Disaster Pharmacy at the University of Geneva, Switzerland. This study tried to determine how hospital pharmacies in Europe are prepared for a natural or manmade disaster and whether they have specifically followed these guidelines since their publication. EAHP reported on this survey in the EU Monitor from 26th January 2018. Based on the outcomes of this survey the Certificate of Advanced Studies (CAS) on “Medicines and Medical Devices in Emergency and Disaster” has been created.
The CAS is seeking to provide a recognised interprofessional post-graduate university training in emergency and disaster pharmacy. Indeed, in crisis contexts, including humanitarian crisis and daily emergencies in (pre-)hospital and hospital settings, the role of drugs and medical devices is obvious in treating victims and guaranteeing the survival of most individuals. In this respect, specific pharmaceutical and pharmacological knowledge is essential for every health professional involved in emergency and disaster situations.
Registration for the CAS is open until 12th January 2020. The program of the CAS will provide qualified pharmacists, physicians, paramedics and other health professionals with new skills and/or updated knowledge, thus strengthening their expertise in this specific field. It will consists of 6 thematic modules of 2-3 days each and a final thesis. The modules will be organised in the Geneva, Lausanne and Bern areas in Switzerland. Courses will be taught mainly in English and international participants are welcome.
Learn more about the CAS on “Medicines and Medical Devices in Emergency and Disaster” HERE
EURORDIS – 10th European Conference on Rare Diseases & Orphan Products
 EAHP has partnered with EURORDIS – the voice of rare disease patients in Europe – for its 10th European Conference on Rare Diseases & Orphan Products (ECRD), recognised globally as the largest, patient-led rare disease event in which collaborative dialogue, learning and conversation takes place, forming the groundwork to shape future rare disease policies. The event will be taking place on 15th and 16th May 2020 at the Stockholmsmässan, in Stockholm (Sweden).
EAHP has partnered with EURORDIS – the voice of rare disease patients in Europe – for its 10th European Conference on Rare Diseases & Orphan Products (ECRD), recognised globally as the largest, patient-led rare disease event in which collaborative dialogue, learning and conversation takes place, forming the groundwork to shape future rare disease policies. The event will be taking place on 15th and 16th May 2020 at the Stockholmsmässan, in Stockholm (Sweden).
ECRD brings together more than 800 actors from more than 50 different countries to discuss innovative solutions in the field of rare diseases. In addition to attending the conference, participants are also invited to submit a poster abstract by 10th February 2020 linked to one of the following themes:
- Theme 1: The future of diagnosis: new hopes, promises and challenges
- Theme 2: Our values, our rights, our future: shifting paradigms towards inclusion
- Theme 3: Share, Care, Rare: Transforming care for rare diseases by 2030
- Theme 4: When therapies meet the needs: enabling a patient-centric approach to therapeutic development
- Theme 5: Achieving the triple A’s by 2030: Accessible, Available and Affordable Treatments for people living with a rare disease
- Theme 6: The digital health revolution: hype vs. reality
- Theme 7: Rare Disease Patient Groups Innovations
- Theme 8: Other/open topic
Learn more about and register for ECRD HERE
New FIP Strategic Plan released

The International Pharmaceutical Federation (FIP) has published its 2019 to 2024 Strategic Plan which was adopted by the FIP Council at its last meeting in September. The Strategic Plan touches on 6 outcomes that should be achieved by the organisation and its members in the coming 5 years.
The strategic outcomes, which will guide the work of FIP, include:
- Everyone has access to the medicines they need.
- Everyone has access to the health and medicines-related information they need.
- Everyone benefits from innovations in medicines, health technologies and services.
- Pharmacists ensure the responsible and quality use of medicines.
- Work collaboratively to ensure comprehensive and integrated healthcare outcomes for patients.
- FIP is a cost-effective, unified, vibrant and growing organisation that meets the needs and supports the work of its members.
Read more about FIP’s Strategic Plan HERE
Stakeholder Network shaping the discussions on AMR

On 18th November 2019 – on the occasion of the European Antibiotic Awareness Day – a new roadmap to tackle antimicrobial resistance was released by the Antimicrobial Resistance (AMR) Stakeholder Network. The roadmap underlines the urgency with which the growing public health threat of AMR needs to be addressed by putting forward strategies through which stakeholders can help tackle the problem.
The AMR Stakeholder Network brings togethers 80 different civil society and healthcare professional organisations and individuals committed to combatting AMR at national, regional and European level from a ‘One Health’ approach. It is hosted within the European Commission’s Health Policy Platform and led by the European Public Health Alliance (EPHA).
The signatories of the Roadmap urge the EU institutions and/or Member State governments to
- set targets and performance indicators to measure progress
- help countries mobilise resources for better implementation of national AMR policies
- close the existing collaboration gap between civil society and EU policy-makers
- put prevention at the heart of AMR policy-making
- tackle the environmental dimension of AMR in the framework of the European Green Deal
In addition to the roadmap, EPHA also spearheaded the creation of an interest group on antimicrobial resistance which has been joined by twelve Members of the European Parliament (MEPs). Biljana Borzan, Sara Cerdas, Olivier Chastel, Petra de Sutter, Fredrick Federley, Roberta Mentsola, Sirpa Pietikäinen, Christel Schaldemose, Tomislav Sokol, Nicolae Ştefănuţă, Sarah Wiener and Tiemo Wölken have committed themselves to ensuring that AMR remains high on the EU policy agenda during the next 5 years.
Read the roadmap of the AMR Stakeholder Network HERE
Learn more about the MEP Interest Group on AMR HERE
EJHP: Intravenous drug use in neonatal intensive care units

The online first edition of the European Journal of Hospital Pharmacy (EJHP) recently published an original article summarising the outcomes of a study that looked at intravenous drugs most frequently used in Spanish Neonatal Intensive Care Units (NICU), their preparation and the implementation rate of standardised concentration infusions. Intravenous drug use in NICUs was identified as a high-risk process and error-reduction strategies, such as concentration standardisation, have been recommended. It was concluded that further data are necessary to design the most suitable intervention in Spain and that institutional initiatives are needed to achieve this.
Read the article HERE
Have you met EAHP’s Statement Implementation Ambassadors?
To promote the implementation of the European Statements of Hospital Pharmacy at national level EAHP has teamed up with motivated and enthusiastic hospital pharmacists willing to help their countries and others move towards Statement Implementation, the so call “Implementation Ambassadors”. It is their role to
- Act as primary link in between EAHP, their national associations and the implementation activities done within their countries.
- Convey to EAHP the needs and priorities of their countries regarding Statement implementation.
- Build resources that will help hospital pharmacists, healthcare professionals and other relevant stakeholders to implement the Statements within their hospitals.
In case you are curious about the Implementation Ambassador working in your country you can find out more about him/her via the Statement website.
Contribute to EAHP’s 2019 Medicines Shortages Survey
EAHP has released its 2019 Medicines Shortages Survey which seeks to investigate the reasons and impact of medicines shortages on patients in European hospitals as well as possible solutions. Unlike previous surveys, this year’s investigation will target all healthcare professionals working in the hospital environment as well as patients that have experienced medicines shortages during their hospital stay.
Share your views by 13th of January 2020 via one of the following links:
Access the English version HERE
Access the French version HERE
Access the Greek version HERE
Access the Portuguese version HERE
Acces to the Romanian version HERE
Access the Slovak version HERE





