EU MONITOR - Follow one of the EAHP open learning courses
The EAHP EU Monitor is a regular round up of news relevant to hospital pharmacy in Europe.
Use your summer to follow one of EAHP’s online learning courses
 If you are still looking for some interesting online courses to follow during your summer vacation, the European Association of Hospital Pharmacists (EAHP) has an idea for you. Join one of the courses included in EAHP’s open learning environment to advance your skills linked to management, biosimilars, medicines shortage, anticoagulants, drug monitoring or procurement.
If you are still looking for some interesting online courses to follow during your summer vacation, the European Association of Hospital Pharmacists (EAHP) has an idea for you. Join one of the courses included in EAHP’s open learning environment to advance your skills linked to management, biosimilars, medicines shortage, anticoagulants, drug monitoring or procurement.
To support its members all across Europe, EAHP initiated the creation of an open learning environment in spring 2018. Since then 10 different open learning courses have been added ranging from 1.5-hour training on specific topics to 5-hour in-depth courses. Hospital pharmacists and other interested healthcare professionals can choose from the following courses:
- Management and Leadership
- Medicines Shortages – Causation and Approaches to Improvements
- Therapeutic Drug Monitoring as a Tool for Therapy Optimisation
- Anticoagulants - Show me the evidence!
- The essentials of biologicals – past, present and future
- Biosimilars in cancer care - the next challenge
- Anticoagulation - From theory to practice
- Biosimilars in breast cancer - the next challenge
- Facing Brexit and FMD - Is Europe ready for the double “storm”?
- Biosimilars – available yet sometimes missing – the challenge of procurement
EAHP is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education. Participants of the open learning courses will be entitled ACPE credits. These credits can be obtained after the completion of the course and a short survey.
Access EAHP’s Open Learning courses via the following LINK
Save the date – EAHP Congress registration opening on the 1st of August!
 From the 23rd to 25th of March, EAHP is inviting you all to Vienna, Austria to experience the 26th annual congress focusing on “Hospital pharmacists – changing roles in a changing world”. You can already circle the 1st of August in your calendars. Like every year, EAHP will open registration and abstract submission on that date.
From the 23rd to 25th of March, EAHP is inviting you all to Vienna, Austria to experience the 26th annual congress focusing on “Hospital pharmacists – changing roles in a changing world”. You can already circle the 1st of August in your calendars. Like every year, EAHP will open registration and abstract submission on that date.
Learn more about EAHP’s 26th annual congress HERE
New EU strategic framework on health and safety at work
 The European Commission released the EU’s newest strategic framework on health and safety at work focusing on managing change brought by green, digital and demographic transitions as well as changes to the traditional work environment, improving prevention of accidents and illnesses and increasing preparedness for any potential future crises. The new framework will be applicable until 2027.
The European Commission released the EU’s newest strategic framework on health and safety at work focusing on managing change brought by green, digital and demographic transitions as well as changes to the traditional work environment, improving prevention of accidents and illnesses and increasing preparedness for any potential future crises. The new framework will be applicable until 2027.
Occupational health and safety (OSH) is curial for protecting workers' health, the functioning of society and continuity of critical economic and social activities. The strategic framework will help to mobilise EU institutions, Member States and social partners around common priorities on workers' protection to improve their health and safety over the coming years. The actions in the strategic framework will be implemented through strong social dialogue, strengthened evidence-based policy-making, improved enforcement and monitoring of existing EU legislation, awareness-raising and mobilising funding to invest in occupational safety and health. Member States are asked to update their national occupational safety and health strategies in line with the new EU framework to ensure that the measures envisioned reach the workplace.
Access the strategy and its accompanying documents HERE
Slovenia takes over Council Presidency
 On 1 July 2021, Slovenia took over the presidency of the Council of the European Union. Under the slogan "Together. Resilient. Europe.", Slovenia wants to build in the next 6 months on the work of German and Portugal to support the EU’s recovery from the COVID-19 crisis.
On 1 July 2021, Slovenia took over the presidency of the Council of the European Union. Under the slogan "Together. Resilient. Europe.", Slovenia wants to build in the next 6 months on the work of German and Portugal to support the EU’s recovery from the COVID-19 crisis.
In the field of health, the presidency will put focus on EU level cooperation. Finding and implementing innovative solutions for resilient health systems, advancing the role of the EU in global health and the European Cancer Plan, improving crisis preparedness and response mechanisms, starting the discussion on a legislative proposal to establish a new Health Emergency Response Authority (HERA), continuing discussion on new solutions at EU level, which have the potential to improve the accessibility or availability of medicines and addressing issues of medicines shortages will play a key role.
Read the programme of the Slovenian Council Presidency HERE
Learn more about the Slovenian Council Presidency’s activities HERE
Commission launches Knowledge Centre on Cancer
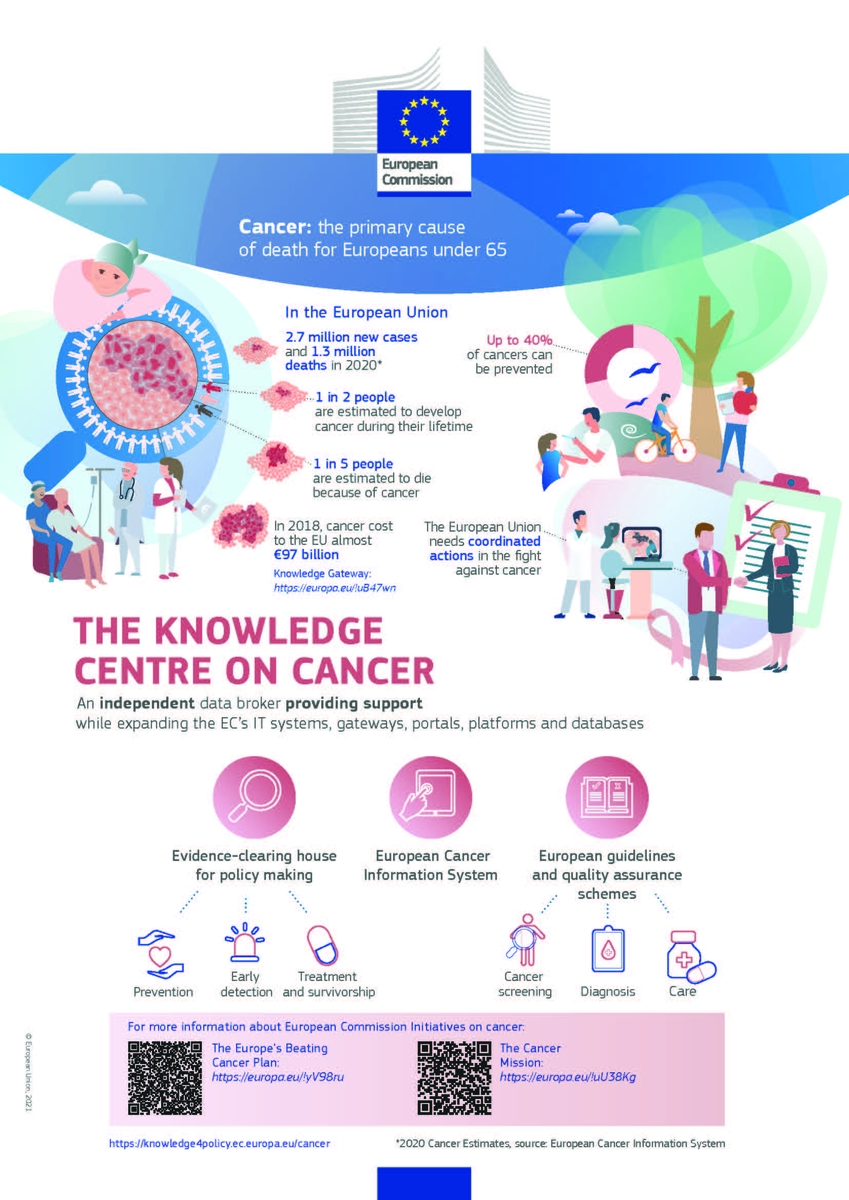 At the end of June, the European Commission launched the Knowledge Centre on Cancer, the first Flagship action delivered under Europe's Beating Cancer Plan. This new online platform collects evidence and coordinates actions against the number one cause of death among under-65s in Europe.
At the end of June, the European Commission launched the Knowledge Centre on Cancer, the first Flagship action delivered under Europe's Beating Cancer Plan. This new online platform collects evidence and coordinates actions against the number one cause of death among under-65s in Europe.
As an independent knowledge broker, the Knowledge Centre on Cancer provides evidence-based support to policies, while expanding the European Commission's existing IT systems, gateways, portals, platforms and databases on cancer. It will:
- Map and provide the latest evidence and statistics on cancer;
- Monitor cancer trends so that the effectiveness of preventive strategies and screening programmes can be evaluated;
- Provide European guidelines for cancer prevention, screening, diagnosis and care to improve cancer outcomes and reduce inequalities between EU regions;
- Help shape policies for cancer prevention related to the environment and healthy lifestyles, including tobacco and alcohol control;
- Identify research or policy gaps;
- Provide a space to coordinate many cancer initiatives on one platform; and
- Help to reduce inequalities in cancer prevention and care across the EU.
Access the Knowledge Centre on Cancer HERE
New report shows that the use of antibiotics in animals is decreasing
 The third joint inter-agency report on integrated analysis of antimicrobial agent consumption and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals (JIACRA) has been published. Work carried out by the European Centre for Disease Prevention and Control (ECDC), the European Food Safety Authority (EFSA), and the European Medicines Agency (EMA) showed that antibiotic use is now lower in food-producing animals than in humans.
The third joint inter-agency report on integrated analysis of antimicrobial agent consumption and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals (JIACRA) has been published. Work carried out by the European Centre for Disease Prevention and Control (ECDC), the European Food Safety Authority (EFSA), and the European Medicines Agency (EMA) showed that antibiotic use is now lower in food-producing animals than in humans.
The report from the three EU agencies looks at data on antibiotic consumption and the development of antimicrobial resistance (AMR) in Europe for 2016-2018. It suggests that the decline in the use of antibiotics in animals is linked to the effectiveness of measures taken at country level to reduce use. Consumption of antibiotics included in the class called polymyxins, which also includes colistin, nearly halved between 2016 and 2018 in food-producing animals. For use of carbapenems, 3rd- and 4th-generation cephalosporins and quinolones in humans the report shows an association with resistance to these antibiotics in Escherichia coli infections in humans. Similar associations were found for food-producing animals.
The report also identifies links between antimicrobial consumption in animals and AMR in bacteria from food-producing animals, which in turn is associated with AMR in bacteria from humans. An example of this is Campylobacter spp. bacteria, which are found in food-producing animals and cause foodborne infections in humans. Experts found an association between resistance in these bacteria in animals and resistance in the same bacteria in humans.
Read the report HERE
Consultation on EU4health related priorities, strategic orientations and needs
 The European Commission is looking for feedback from stakeholders on the priorities, strategic orientations and needs to be addressed through the EU4Health annual work programme for 2022. The consultation will close on 31 July 2021.
The European Commission is looking for feedback from stakeholders on the priorities, strategic orientations and needs to be addressed through the EU4Health annual work programme for 2022. The consultation will close on 31 July 2021.
EU4Health is the EU’s ambitious response to COVID-19. The pandemic has a major impact on patients, medical and healthcare staff, and health systems in Europe. The new EU4Health programme will go beyond crisis response to address healthcare systems’ resilience in relation to ten specific objectives falling under the 4 general goals of the programme:
- To improve and foster health in the Union
- disease prevention & health promotion
- international health initiatives & cooperation
- To tackle cross-border health threats
- prevention, preparedness & response to cross-border health threats
- complementing national stockpiling of essential crisis-relevant products
- establishing a reserve of medical, healthcare & support staff
- To improve medicinal products, medical devices and crisis-relevant products
- making medicinal products, medical devices and crisis-relevant products available and affordable
- To strengthen health systems, their resilience and resource efficiency
- strengthening health data, digital tools & services, digital transformation of healthcare
- improving access to healthcare
- developing and implementing EU health legislation and evidence-based decision making
- integrated work among national health systems
Access the targeted consultation HERE

EJHP: Read the July issue!
The latest issue of the European Journal of Hospital Pharmacy (EJHP) is available. The editorial presents the CLEO assessment tool for pharmacist interventions, a systematic review that evaluates the type, incidence and risks of dermatological toxicities from dabrafenib, several original research articles, short and case reports and electronic pages. Among other things, the July issue gathers the EAHP’s needs assessment survey and the public consultation for the European health data space.
Read the July issue HERE
 [COVID-19 Updates]
[COVID-19 Updates]
EAHP’s COVID-19 Resource Centre
To assist its member associations and individual hospital pharmacists in this critical time with the provision of the best possible care for patients, EAHP has decided to gather and make available information on COVID-19 relevant for the hospital pharmacy profession.
Access the Resource Centre HERE
French High Council for Public Health - COVID-19: treatment recommendations antagonists of IL1 and IL6 | COVID-19 : recommandations thérapeutiques antagonistes des IL1 et IL6
The High Council of Public Health (HCSP) updated the recommendations relating to the prescription of antagonists of IL1 and IL6 receptors in the treatment of COVID-19.
Read the recommendations HERE
NICE - SYNE-COV for predicting COVID-19 outcomes
SYNE-COV is a cloud-based software designed to help manage COVID‑19 in hospitals. It predicts the chance of people with COVID‑19 being admitted for intensive care, having mechanical ventilation, or dying while in hospital.
Learn more HERE
Journal of Pharmaceutical Analysis - Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine, serum and saliva samples by GC-MS
The study assesses several liquid phase microextraction and solid phase extraction methods to determine the most efficient method for the preconcentration of hydroxychloroquine sulfate.
Read the article HERE
Journal of Pharmacy Practice - Hospital Pharmacy Response to Covid-19 Pandemic in Italy: What We Learned From the First Outbreak Wave
The aim of this study was to describe the impact of COVID-19 emergency on pharmaceutical care provided by pharmacists during the first wave of the pandemic.
Read the article HERE
Have you completed your SAT yet?

To help you with the implementation of the European Statements of Hospital Pharmacy, EAHP has developed a self-assessment tool (SAT) which helps you understand the level of Statement implementation in your pharmacy. The tool has been rolled out across Europe. Different language versions (Czech, English, French, German, Greek, Hungarian, Italian, Polish, Portuguese, Romanian, Serbo-Croatian, Spanish and Turkish) have been made available to enable as many hospital pharmacists as possible to use the tool. Talk to your chief pharmacist and encourage him/her to work with the SAT. In case you are the head of the pharmacy get your team together and complete your assessment today with the help of the SAT.
Learn more about SAT HERE
___________________________________________________________________________________
![]()
Consultations
European Commission – Public Consultation: Digital health data and services – the European health data space
To ensure that all possible views are considered in the design of a legal framework for a European Health Data Space (EHDS), the European Commission invites all interested individuals and stakeholders to share their views and experiences. Your contribution can help provide important insights, opinions and evidence to support the impact assessment accompanying the EHDS proposal on the problems to be tackled, the policy options to be considered and their likely impacts.
Deadline – 26 July 2021
Access the consultation HERE
European Commission – Public Consultation: Cross-border healthcare – evaluation of patients’ rights
The Directive on patients’ rights in cross-border healthcare (2011/24/EU) aims to facilitate access to safe and high quality healthcare in another EU country. The European Commission invites all interested individuals and stakeholders to share their views and experience in seeking planned healthcare across the EU or in EU cooperation in the area of rare diseases.
Deadline – 27 July 2021
Access the consultation HERE
European Commission – Public Consultation: Medicines for children & rare diseases – updated rules
This initiative will explore several options to address the shortcomings identified in the evaluation of the Regulations on medicines for children and rare diseases, in view of a revision of the existing legislation. With this public consultation, citizens and stakeholders are invited to share their views and experiences on the main obstacles they are facing concerning treatments for rare diseases and children, on possible ways to overcome these obstacles and on how to make the current legislation future-proof.
Deadline – 30 July 2021
Access the consultation HERE




